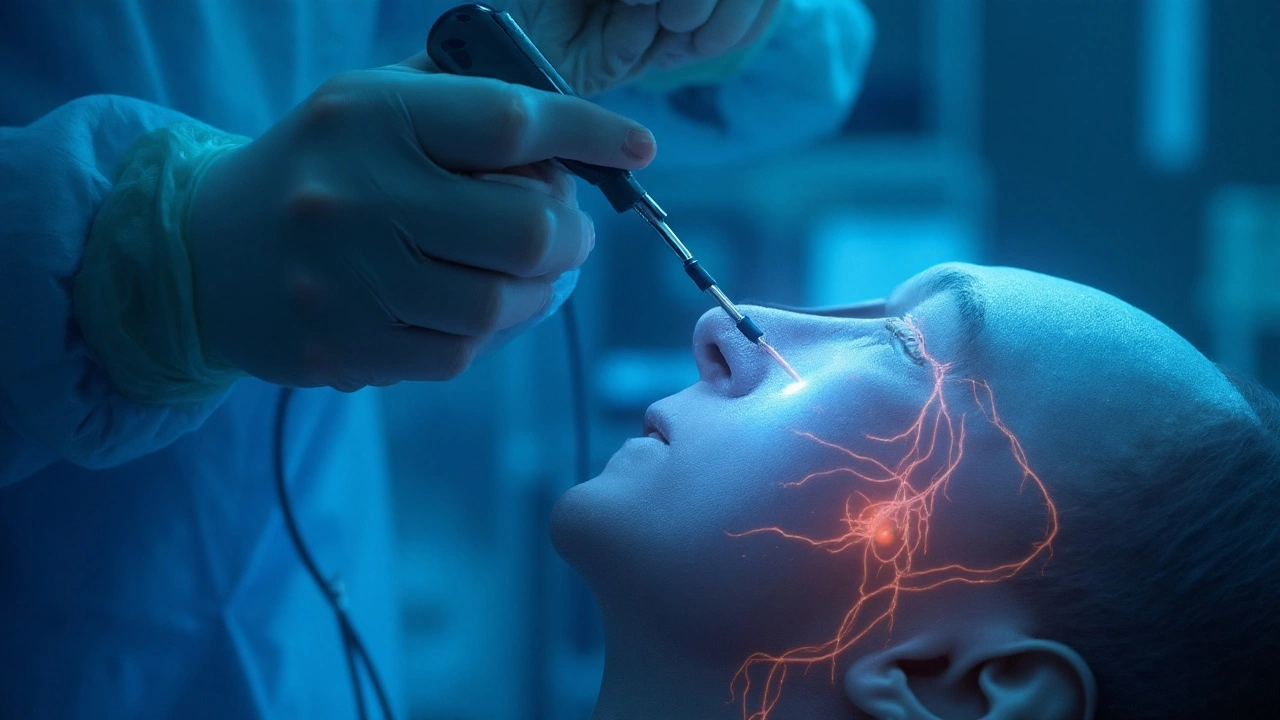
Deep Brain Stimulation is a surgical therapy that delivers continuous electrical pulses to specific brain nuclei to modulate abnormal neural activity. For people living with Parkinsonism a syndrome that includes Parkinson’s disease, multiple system atrophy and related movement disorders, DBS can smooth out tremor, stiffness and the dreaded “off” periods that make daily life unpredictable.
Why Consider DBS? A Quick Snapshot
- Reduces motor symptoms by 30‑60% on average (data from multicenter trials 2022‑2024).
- Lowers required levodopa dose, cutting medication‑induced dyskinesia.
- Improves quality‑of‑life scores on the Parkinson’s Disease Questionnaire‑39 (PDQ‑39) by 15‑20 points.
These numbers mean many patients can return to hobbies, work, or simply enjoy a quieter evening without constant shaking.
How the System Works
The DBS set‑up consists of three core components:
- Implantable pulse generator (IPG) a battery‑powered device placed under the collarbone that creates programmable electrical pulses.
- Leads (electrodes) thin insulated wires that travel from the IPG into the brain target.
- Programmer a handheld console used by neurologists to fine‑tune voltage, pulse width and frequency.
During surgery, the neurosurgeon places the leads into a pre‑selected nucleus-most commonly the Subthalamic Nucleus (STN) a small almond‑shaped structure that regulates motor output or the Globus Pallidus Internus (GPi) the output hub that suppresses unwanted movements. The choice of target shapes the clinical profile, as shown in the table below.
| Attribute | STN | GPi |
|---|---|---|
| Primary benefit | Greater reduction in levodopa dose | Better control of dyskinesia |
| Typical motor improvement (UPDRS‑III) | 45‑55% off medication | 35‑45% off medication |
| Common side‑effects | Speech and cognitive slowing | Mood changes, more surgical time |
| Battery life (IPG) | 3‑5 years (standard) | 4‑6 years (standard) |
Who Benefits Most? Candidate Checklist
Not everyone with Parkinsonism is a good fit. The following criteria, drawn from the National Institute for Health and Care Excellence (NICE) 2023 guidelines, help clinicians decide:
- Diagnosed with Parkinson’s disease (or Parkinsonism) for at least 4years.
- Experiences motor fluctuations despite optimized oral therapy.
- Has disabling tremor or rigidity that interferes with daily tasks.
- Levodopa‑induced dyskinesia that limits functional ability.
- Age generally between 45 and 75years (younger patients often see longer battery life).
- No severe uncontrolled psychiatric illness, active infection, or contraindicating clotting disorder.
When a patient meets most of these points, a multidisciplinary team (neurologist, neurosurgeon, neuropsychologist, physiotherapist) conducts a formal assessment.
The Surgical Journey - What to Expect
1. Pre‑operative work‑up: Brain MRI, CT‑guided stereotactic planning, and a neuropsychological battery to establish baseline cognition.
2. Stage1 - Lead implantation: Under local anaesthetic, a thin skull opening is made. The patient stays awake so the team can test symptom relief in real time.
3. Stage2 - IPG placement: Usually a day later, under general anaesthetic, the pulse generator is tucked under the collarbone.
4. Programming phase: Starts a few weeks post‑op. Initial settings are conservative; weekly or bi‑weekly visits adjust parameters until optimal control is achieved.
5. Long‑term follow‑up: Battery checks every 6‑12months, hardware inspections, and periodic re‑programming to adapt to disease progression.
Device Landscape - Leading Manufacturers
Two major companies dominate the European market:
- Medtronic Activa the longest‑standing system, offering both rechargeable and non‑rechargeable IPGs.
- Boston Scientific Vercise known for directional leads that focus current, reducing side‑effects.
Clinical trials in 2023 showed Vercise’s directional leads shaved 0.5seconds off reaction‑time tasks compared with traditional ring leads, while Activa’s rechargeable battery can last up to 9years.

Benefits Beyond Motor Control
DBS isn’t just about stopping tremor. Emerging evidence (2022‑2024) links stimulation to:
- Reduced depression scores when the target is the ventral STN, likely via limbic circuit modulation.
- Improved gait and freezing of gait especially with combined STN and pedunculopontine nucleus (PPN) stimulation.
- Cognitive stability in well‑selected patients, as long as stimulation parameters avoid excessive spread to the zona incerta.
These broader effects make DBS a holistic tool, not just a tremor‑killer.
Risks, Complications, and How to Mitigate Them
Every surgery carries risk. For DBS, the most common issues are:
- Hardware‑related: lead fracture (≈2% per year) or IPG migration.
- Neurological: transient speech slowing, mood swings, or mild confusion.
- Infection: scalp or chest pocket infection rates around 3‑5%.
Strategies to keep complications low include meticulous sterile technique, using directional leads to fine‑tune current, and early neuropsychological monitoring. If an infection does occur, prompt antibiotics and, if needed, hardware removal usually resolve the issue.
Living with DBS - Real‑World Stories
John, a 62‑year‑old former electrician from Manchester, struggled with “off” periods that left him unable to finish a simple wiring job. After STN DBS, his daily “off” time dropped from 6hours to under 1hour. He now volunteers at a local community centre, teaching DIY classes again.
Maria, 58, had severe levodopa‑induced dyskinesia that made writing impossible. She opted for GPi DBS; within three months, her dyskinesia score fell by 70%, and she could sign her name without shaking.
These accounts illustrate how the right target, patient selection, and programming turn a complex surgery into a life‑changing therapy.
Future Directions - Adaptive DBS and Beyond
Traditional DBS delivers constant stimulation. Adaptive or closed‑loop DBS, emerging from trials in 2024, uses sensed brain signals (like beta‑band activity) to automatically adjust voltage. Early data show a further 15‑20% reduction in motor fluctuations and a 30% increase in battery longevity.
Other investigational avenues include focused ultrasound lesioning as a non‑implant alternative, and combination therapy with gene‑editing techniques to address the underlying neurodegeneration.
Frequently Asked Questions
How long does DBS surgery take?
The lead implantation phase usually lasts 2‑3hours, while the IPG placement adds another 1‑2hours. Total operative time is typically under 5hours.
Can DBS be turned off if side‑effects appear?
Yes. The programmer can instantly reduce or stop stimulation, allowing clinicians to identify whether a symptom is stimulation‑related and then re‑program to a safer setting.
Is DBS covered by the NHS?
In England, Scotland and Wales, DBS for Parkinson’s disease that meets NICE criteria is funded by the NHS. Private clinics also offer the procedure for patients who wish to avoid waiting lists.
How often must the battery be replaced?
Non‑rechargeable IPGs need surgical replacement every 3‑5years, depending on usage. Rechargeable models can last 9‑12years and are recharged transcutaneously at home.
Will DBS cure Parkinson’s disease?
No. DBS manages symptoms but does not halt neurodegeneration. Patients still need medication and regular follow‑up to address disease progression.




Adrianna Alfano
September 22, 2025 AT 13:43i just saw my aunt go through this last year and honestly i didnt think shed ever hold a cup again without spilling it everywhere now she can water her plants and even knit again. the way her hands stopped shaking was like watching a ghost come back to life
May .
September 23, 2025 AT 08:36dbS works but its not magic
Josh Bilskemper
September 24, 2025 AT 07:04you people are overhyping this like its a cure when the real issue is the pharmaceutical industry pushing implants because pills are less profitable
Jessica Ainscough
September 24, 2025 AT 11:02my dad had this done two years ago and the biggest change was not the tremors but the fact that he could finally sleep through the night. before he was up every hour twitching or stiff. now he watches late night tv like a normal person. it’s not just about movement its about dignity
Sara Larson
September 25, 2025 AT 09:31THIS IS SO LIFE CHANGING 🥹 my uncle went from needing help to stand to hiking with his grandkids. if you’re even considering it DO IT. the fear is worse than the surgery
Cristy Magdalena
September 25, 2025 AT 17:30you all are being so naive. sure the device works but have you considered the psychological toll of having a machine permanently wired into your brain? it’s not just a pacemaker for your motor cortex. it’s a permanent alteration of your neural identity. i’ve seen patients become emotionally flat after STN stimulation. they say they’re ‘better’ but they’re not the same person. and who’s to say the long term cognitive effects aren’t just being swept under the rug because the industry makes billions off this?
the data looks good on paper but real life is messy. one woman i know cried for weeks after her programming session because she forgot what it felt like to be ‘bad’ - to have the shaking, the struggle, the humanity of it all. now she’s ‘perfect’ but hollow.
and dont get me started on the battery replacements. imagine having open brain surgery every 4 years because a lithium ion cell dies. that’s not progress. that’s a corporate treadmill dressed up as medicine.
they say adaptive DBS is the future. fine. but what if the algorithm learns to suppress your sadness along with your tremor? what if it optimizes for compliance not consciousness? we’re not just treating disease anymore. we’re editing personality.
and yes i know i sound dramatic. but someone has to say it while everyone else is clapping for the glowing screen that controls their loved one’s soul.
Storz Vonderheide
September 26, 2025 AT 20:31just wanted to add that in some rural clinics in the midwest they’re starting to use telehealth for programming now. you don’t have to drive 3 hours to the neuro center every month. your neurologist can adjust settings over secure video and send updates to the device remotely. it’s a game changer for people without reliable transport.
also if you’re worried about battery life the newer rechargeables are way better than people think. my cousin charges hers once a week for 20 minutes while she watches netflix. no surgery for 10 years. that’s not bad.
dan koz
September 28, 2025 AT 10:03i saw this in nigeria last year. one man had dbS done in a private hospital in lagos. he was dancing at his daughter’s wedding after 8 years of being still. nigeria needs this more than usa. why only rich people get it?
Casey Lyn Keller
September 28, 2025 AT 14:40the fact that medtronic and boston scientific are the only two players makes me wonder if this is more about market control than patient care. no competition means no pressure to lower prices. and the battery replacement costs alone could bankrupt a family without good insurance. this isn’t healthcare. it’s a luxury subscription service with electrodes.