Hypertrophic Subaortic Stenosis vs. Coronary Artery Disease Risk Calculator
Enter your information and click "Calculate Risk" to see your assessment.
Hypertrophic Subaortic Stenosis (HSS)
Can cause LVOT obstruction, leading to symptoms like chest pain, shortness of breath, and fainting. May be masked by CAD symptoms.
Coronary Artery Disease (CAD)
Caused by plaque buildup in coronary arteries, reducing blood flow to heart muscle. Can cause chest pain and heart attacks.
Overlap Risk
Patients with HSS have an increased risk of developing CAD due to shared risk factors and pathophysiology.
When you hear the words hypertrophic subaortic stenosis, you might picture a thickened heart muscle that blocks its own outflow. When you think about coronary artery disease, you probably imagine clogged arteries that starve the heart of blood. What many people don’t realize is that these two conditions often share pathways, can mask each other’s symptoms, and sometimes make treatment trickier than it appears.
What Is Hypertrophic Subaortic Stenosis?
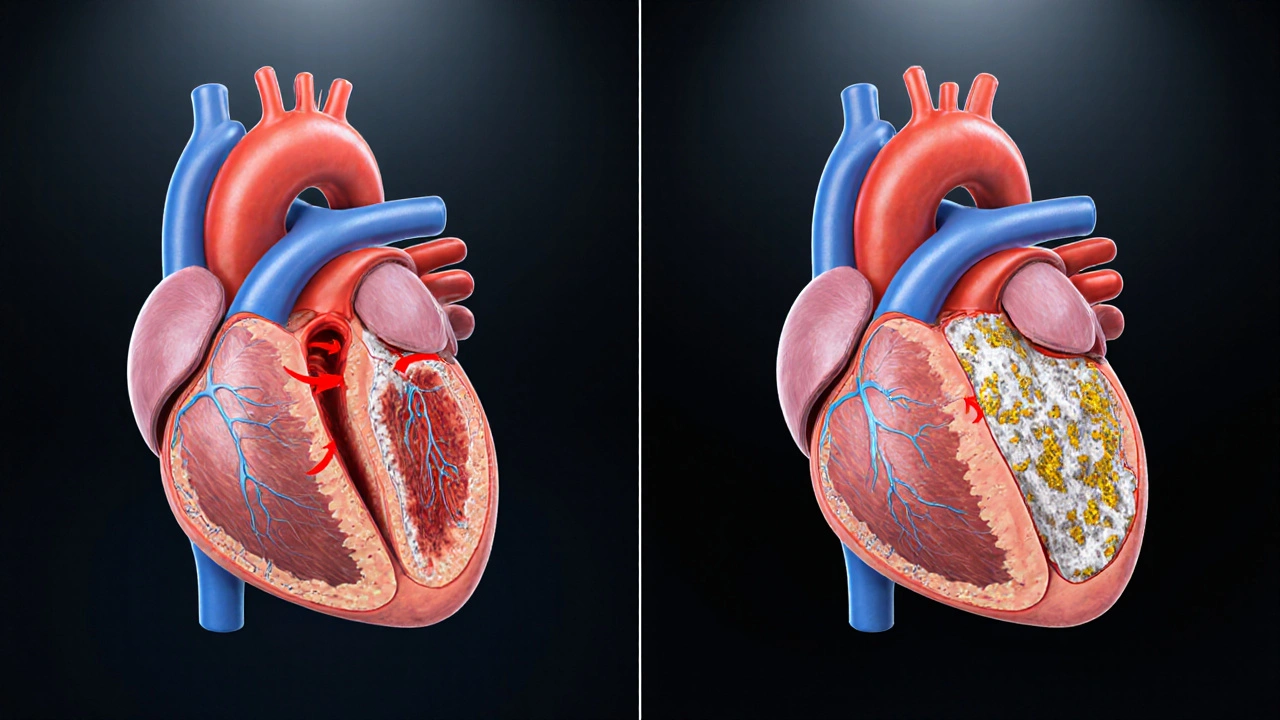
Hypertrophic Subaortic Stenosis is a form of inherited heart muscle disease that causes the ventricular septum to thicken, narrowing the left ventricular outflow tract (LVOT). The condition is also called hypertrophic obstructive cardiomyopathy (HOCM). It affects roughly 1 in 500 people worldwide, and many carriers never notice any problems.
The thickened septum creates a pressure gradient during each heartbeat. The heart works harder to push blood into the aorta, which can lead to shortness of breath, chest discomfort, fainting spells, or even sudden cardiac death in extreme cases.
What Is Coronary Artery Disease?
Coronary Artery Disease is a condition where the coronary arteries become narrowed or blocked by a buildup of plaque, reducing blood flow to the heart muscle. It’s the leading cause of heart attacks globally and is driven by risk factors such as high LDL cholesterol, smoking, hypertension, diabetes, and a sedentary lifestyle.
When the coronary arteries can’t deliver enough oxygen during increased demand-like during exercise or stress-the heart muscle suffers from ischemia, which feels like chest pressure, tightness, or radiating pain.
Why Do These Two Conditions Overlap?
At first glance they seem unrelated: one is a structural muscle problem, the other a vascular blockage. Yet several mechanisms bridge the gap:
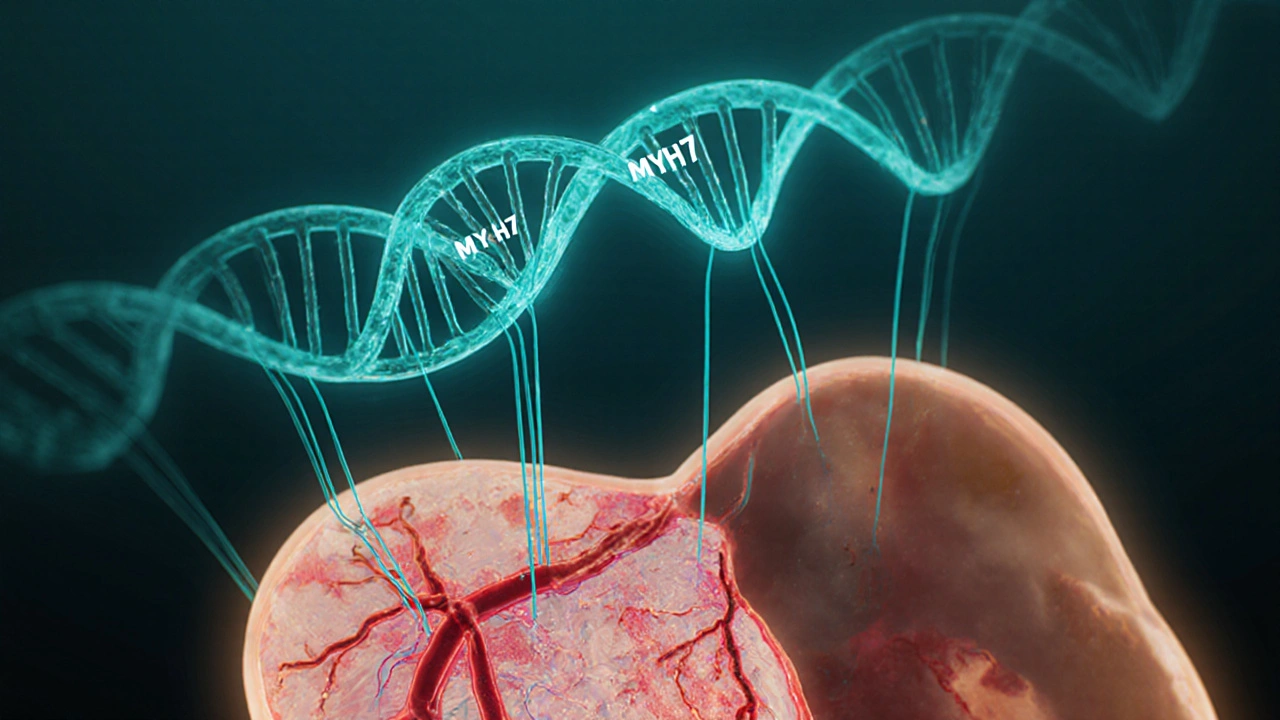
- Shared genetic pathways: Mutations in the MYH7 gene, which encodes a cardiac myosin protein, have been linked to both hypertrophic cardiomyopathy and early‑onset coronary artery disease in some families.
- Microvascular dysfunction: Even without large‑vessel plaque, patients with HSS often have tiny vessel spasms that mimic CAD symptoms. These microvascular changes can evolve into true atherosclerosis over time.
- Increased myocardial oxygen demand: The LVOT obstruction forces the left ventricle to generate higher pressures, raising the heart’s oxygen needs. If coronary supply is already marginal, the imbalance can trigger ischemic chest pain.
- Common lifestyle risk factors: High‑intensity exercise, which some HSS patients love, can raise systolic blood pressure spikes, accelerating plaque formation.
How One Condition Can Mask the Other
Imagine a patient who reports occasional chest tightness after climbing stairs. A physician might order a stress test and see an abnormal response caused by the LVOT gradient, not a blocked artery. Conversely, a person with CAD might attribute exertional dyspnea to blocked vessels when the real issue is a hidden subaortic obstruction.
Because the symptoms overlap-shortness of breath, chest pain, fatigue-accurate diagnosis requires a blend of imaging tools.
Diagnostic Tools That Help Spot Both
Here’s a quick rundown of the most useful tests and how they reveal each condition:
| Test | What It Shows for HSS | What It Shows for CAD |
|---|---|---|
| Echocardiography | Septal thickness, LVOT gradient, systolic anterior motion of mitral valve | Can assess wall motion abnormalities during stress |
| Cardiac MRI | Detailed tissue characterization, late gadolinium enhancement indicating fibrosis | Detects sub‑endocardial scar from prior infarcts |
| Coronary Angiography | Usually normal unless CAD co‑exists | Visualizes plaque, stenosis, and guides stenting |
| Stress Testing (Exercise or Pharmacologic) | May show abnormal blood pressure response due to obstruction | Shows ischemic ST‑segment changes indicating CAD |
When a patient shows abnormal findings on more than one of these tests, clinicians start suspecting a dual diagnosis.
Managing Both Conditions Together
Therapy has to address two fronts: reducing the obstruction from HSS and protecting the coronary arteries from further plaque buildup.
- Beta‑blockers or non‑dihydropyridine calcium channel blockers: These drugs lower heart rate and contractility, easing the LVOT gradient while also decreasing myocardial oxygen demand-a win‑win for both conditions.
- Disopyramide: Often used in HSS to blunt the obstruction. It can also have anti‑arrhythmic benefits for CAD patients prone to ventricular ectopy.
- Statins: Essential for CAD management. Some recent studies suggest statins may modestly improve endothelial function in HSS patients, though the evidence is still emerging.
- Surgical myectomy or alcohol septal ablation: If the LVOT gradient remains high (>50 mmHg) despite medication, removing part of the septum can dramatically improve symptoms. After a successful myectomy, the heart’s oxygen demand drops, making CAD easier to control.
- Lifestyle tweaks: Low‑sodium diet, regular moderate‑intensity aerobic exercise (avoid high‑intensity bursts that raise systolic pressure), and smoking cessation benefit both conditions.
Importantly, any decision about invasive procedures must weigh the patient’s overall risk profile. A combined approach-medication first, then targeted surgery if needed-tends to produce the best outcomes.

Common Pitfalls to Avoid
- Prescribing high‑dose nitrates for chest pain without checking the LVOT gradient. Nitrates can drop preload, worsening obstruction.
- Assuming a normal coronary angiogram rules out ischemia. Microvascular disease linked to HSS can still cause pain.
- Skipping genetic counseling. Family members may carry the same MYH7 mutation and benefit from early screening.
Quick Checklist for Patients and Clinicians
- Ask about family history of HSS or unexplained sudden death.
- Document any chest pain that occurs with low‑intensity activity.
- Order a baseline echocardiogram with Doppler to measure the LVOT gradient.
- Check lipid panel and start statin therapy if LDL>100mg/dL or if plaque is present.
- Review medication list for agents that may exacerbate obstruction (e.g., diuretics, high‑dose nitrates).
- Schedule cardiac MRI if echocardiogram shows ambiguous fibrosis.
- Consider referral to a specialized HSS center for myectomy evaluation if gradient >50mmHg on two separate studies.
Looking Ahead: Research Trends
Two exciting areas are shaping future care:
- Gene‑editing trials: Preliminary CRISPR work on MYH7 mutations shows promise in animal models. Human trials could arrive by 2030.
- Novel microvascular drugs: Agents targeting endothelial nitric oxide pathways are in PhaseII trials and may specifically help HSS patients with hidden coronary dysfunction.
Staying updated on these advances can help patients and clinicians make smarter choices as new therapies become available.
Frequently Asked Questions
Can hypertrophic subaortic stenosis cause a heart attack?
HSS itself doesn’t create plaque, but the high pressure the heart works against can lower coronary flow enough to mimic a heart attack. If a patient also has CAD, the combined stress can trigger a real infarction.
Should I get a stress test if I’ve been diagnosed with HSS?
Yes, but the test should be tailored. Pharmacologic stress with dobutamine can safely reveal how the obstruction behaves under pressure without risking a dangerous blood‑pressure drop.
Are beta‑blockers safe for someone with both HSS and CAD?
They are often the first‑line choice because they slow the heart, reduce the gradient, and lower the heart’s oxygen demand-benefiting both conditions.
What lifestyle changes matter most?
Quit smoking, keep LDL cholesterol below 100mg/dL, stay active with moderate aerobic exercise (like brisk walking or cycling), and limit high‑intensity bursts that raise systolic pressure above 180mmHg.
Is genetic testing worth it?
If you have a family history of HSS, sudden cardiac death, or unexplained heart failure, testing for MYH7 and other sarcomere gene mutations can guide screening for relatives and inform treatment choices.




rajendra kanoujiya
October 8, 2025 AT 21:26I’ve seen too many patients rely on that shiny risk calculator and think it replaces a proper work‑up. In reality you still need an echo, stress test, and a conversation with a cardiologist.
Caley Ross
October 12, 2025 AT 08:46Those risk numbers are useful but they don’t capture the nuance of LVOT gradients.
John Nix
October 15, 2025 AT 20:06While the presented algorithm is methodical, it remains essential to interpret its output within the broader clinical context, acknowledging individual variability.
Kitty Lorentz
October 19, 2025 AT 07:26i think its really helpful how they listed the checklist but sometimes the wording is a bit off like "low‑sodium diet" could be more specific and the part about nitrates is crucial dont forget that
inas raman
October 21, 2025 AT 14:59Totally get you – the checklist is solid, just remember to personalize the diet advice for each patient’s cultural background and activity level.
Jenny Newell
October 25, 2025 AT 02:19The algorithm integrates traditional Framingham variables with HSS‑specific hemodynamic parameters, effectively creating a hybrid risk stratification model.
Kevin Zac
October 27, 2025 AT 08:53Exactly, and when we talk about hemodynamics we should also factor in diastolic dysfunction metrics to fine‑tune the risk profile.
Stephanie Pineda
October 30, 2025 AT 20:13Reading through the whole guide reminded me why interdisciplinary care is the backbone of managing HSS‑CAD overlap.
First, the emphasis on low‑dose beta‑blockers aligns with what we see in the electrophysiology literature, where rate control eases both gradient and myocardial oxygen demand.
Second, the stress‑test recommendation to use dobutamine rather than exercise is not just a safety tweak but a diagnostic lever that can unmask latent ischemia.
Third, the nutritional advice goes beyond the generic low‑sodium mantra, prompting clinicians to consider potassium‑rich foods that support vascular tone.
Fourth, the discussion about myectomy timing is spot on; waiting until the LVOT gradient exceeds 50 mmHg on two studies has become a de‑facto threshold.
Fifth, the caution about high‑dose nitrates is a reminder that classic CAD therapies can paradoxically worsen obstruction in HSS.
Sixth, the suggestion to screen family members for MYH7 mutations reflects the growing trend toward precision cardiology.
Seventh, the mention of emerging CRISPR trials hints at a future where we might correct the sarcomeric defect before it manifests clinically.
Eighth, the microvascular drug pipeline offers hope for patients who suffer angina despite clear epicardial arteries.
Ninth, integrating cardiac MRI into the work‑up provides tissue characterization that can differentiate fibrosis from hypertrophy.
Tenth, the checklist’s step about reviewing medication lists for diuretics underscores the delicate fluid balance in these patients.
Eleventh, the lifestyle section wisely advises moderate aerobic exercise, steering clear of high‑intensity sprints that spike systolic pressure.
Twelfth, the advice to keep LDL below 100 mg/dL dovetails with recent AHA guidelines for secondary prevention.
Thirteenth, the patient‑education points about chest pain patterns empower individuals to seek timely care.
Finally, keeping all these pieces together requires a coordinated team-cardiologists, genetic counselors, and cardiac surgeons-working in sync.
Anne Snyder
November 2, 2025 AT 03:46Great summary, especially the point about avoiding high‑intensity bursts that could push the gradient over the edge.
McKenna Baldock
November 5, 2025 AT 15:06When we consider the heart as a biomechanical organ, the interplay between structural obstruction and vascular pathology becomes a microcosm of the body’s broader struggle between form and function.
Roger Wing
November 7, 2025 AT 22:39Or maybe the whole thing is a placebo engineered by pharma to sell more drugs
Matt Cress
November 10, 2025 AT 06:13Sure, because the government secretly monitors every echo machine and decides who gets a myectomy – classic.
Andy Williams
November 13, 2025 AT 17:33The guideline’s recommendation to maintain LDL < 100 mg/dL is consistent with the 2018 ACC/AHA cholesterol consensus, which categorizes HSS patients with CAD as high‑risk.
Paige Crippen
November 16, 2025 AT 01:06Hidden cardiac labs have been manipulating risk scores for decades to push invasive procedures.
sweta siddu
November 19, 2025 AT 12:26Love the checklist – super handy! 😊
Ted Mann
November 21, 2025 AT 19:59It’s fascinating how a simple list can become a bridge between patient autonomy and clinical expertise, reminding us that medicine is as much art as science.